The Cell as an Engine
NEW 2019
What is an Engine?
Any system capable of doing thermodynamic work is an engine. Work in this sense is the organisation of energy into some sort of coherent action (see below). Engines pump entropy from a coupled system into passing energy. The net result is dissipation of the organisation of the passing energy and an increase in organisation of the coupled system. This organisation might be a macroscopic movement such as your car going along the ground (as opposed to just getting hot or breaking apart), or it might be the assembly of a specific bio-molecule, or machine part. All engines operate by providing a constraining path for energy to flow - from low entropy to high entropy states. Often (but not always) this entails a change of the medium in which the energy is embodied. For example, in a combustion engine, chemical energy (that stored in chemical bonds, e.g. in Diesel fuel), is transferred into mechanical (kinetic) energy as a rotor turns; this might be transferred into an electrical (electron / positron gradient) embodiment of energy via a dynamo and the resulting flow of electrons might drive an electric motor, passing the energy onto a mechanical embodiment again. There would be three engines involved in that chain and at every stage, indeed always and under every circumstance, the total energy does not change. Energy is never used up, engines, cells, you and I, never consume energy, we all just pump our excess entropy into the energy that passes through us (this is the lesson of the 1st law of thermodynamics).
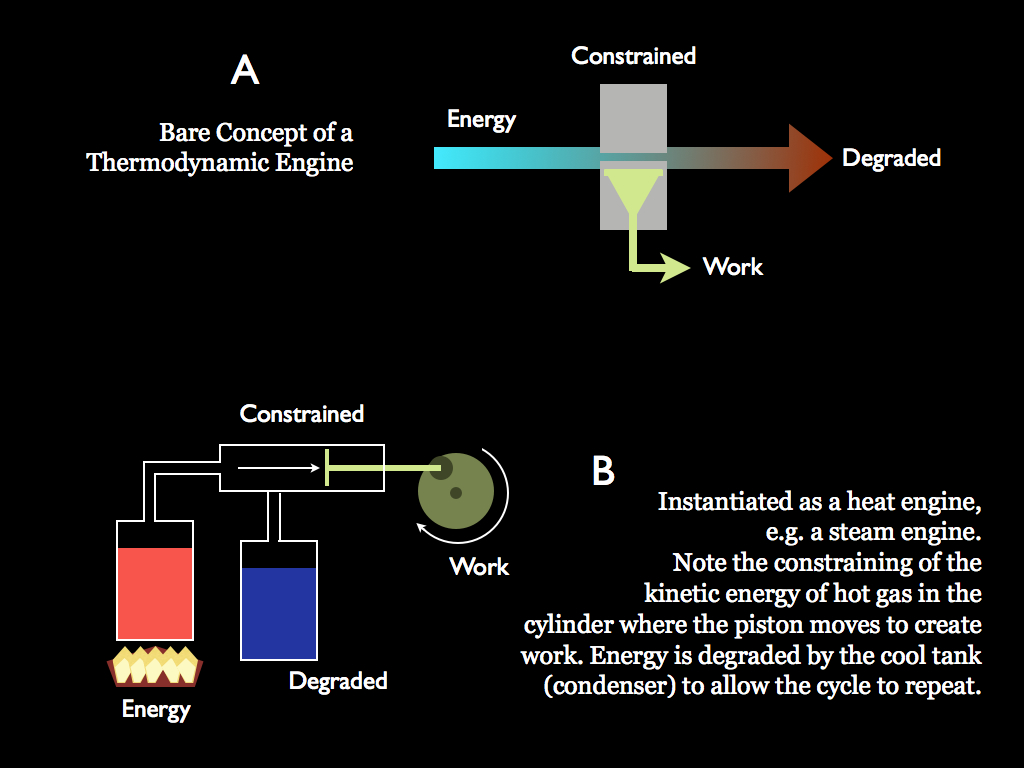
In diagram A, we see the bare bones of an engine. Energy flows through it and as it does, the energy is constrained in some way, so that its degrees of freedom are reduced. It takes negative entropy to do that and this is provided by information that is embodied in the shape of the engine components. The constraint of the energy is what enables it to do work by acting, to some extent, coherently. In an engine, some of the energy is transferred into a coupled system, this is the part that is acting coherently and with its coherence it is carrying negative entropy into the coupled system. What is left is the rest of the energy which now has more entropy than it had before entering the engine (it gained entropy in balance of that which left with the coherent energy). Well, this is a bit abstract, so it is good to refer to the example in diagram B.
Here a simple heat engine illustrates these processes. The turning wheel is the coupled system. Energy is released by burning fuel - this free energy is heat, which really means it is added kinetic energy in the molecules of the hot gas (them moving about faster is what we interpret as hot). They are all going in random directions, but when they go into the cylinder with its piston, they are rather constrained by the sides and create pressure, including against the piston. The sides don't move (if all is working well), so the only expression of the free energy allowed by the constraints is movement in the direction of pushing the piston. The random movements of the hot gas are directed into coherent pushing of the piston. The resultant movement is what turns the wheel. But it's not over yet: that would be a very short action and not much use, unless the piston could return to the left and let the whole thing happen again. Once the hot gas has done its work and the piston is over to the right, cold gas is introduced to cool the hot gas, slowing down its molecular constituents, reducing the pressure in the cylinder and letting it run back to the left, driven by the inertia of the wheel. With the piston back over to the left, another influx of hot gas can start the process again. We have a cycle: it is a thermodynamic work cycle. Obviously some valves which admit hot and cold gass at the correct times would be an important addition. These valves are also obviously more constraints and themselves embody information: indeed the whole physical design of the engine is a set of constraints made from information embodied by the engine itself.
Not all engines need to perform cycles: the most basic criterion for an engine is the extraction of work from passing energy. An obvious example is the water wheel, or windmill - the kinetic energy of a river or the wind is made to do work by constraining it to push against flaps or blades as it goes by. If you connect a battery (a chemical energy cell) to a suitable electric motor, then the flow of electrons driven by the chemical reaction in the battery would turn the motor by electro-motive force. Inside the battery, a substance that acts as an electron donor supplies electrons to one that acts as an electron acceptor (though it is probably not individual electrons that move, rather, this is the net effect of moving charged atoms or small charged molecules. By having a collection of electron donor molecules and separately a collection of electron acceptors, you would have a handy energy source: energy that is ready to flow. This is what your battery is, but more significantly it is also what every cell that ever lived does for its flow of energy.
The Engine in every cell.
Because life is a complex of information-rich machinery that takes an awful lot of negative entropy to create the organisation needed to form it, life must have a source of work as a means of pumping entropy out of itself. In other words, every cell that ever lived has needed a constrained flow of energy from which to extract work. To illustrate this, we will first look at the cell that is among the most like the first ancestor of all cells: the hot spring bacterium species going by the name Aquifex. I have picked this one because it is about the closest we can get (among extant organisms) to the primitive living systems that first appeared as true life and its way of pumping entropy is probably as old as life itself*. Aquifex are also remarkably simple, having the least DNA of any genuinely free living bacterium (there are others with less, but they are parasites, so not really self-reliant).
Imagine for a moment, the Earth 3.8 billion years ago. There is very close to no free oxygen, it is nearly hot enough to boil water and there is an abundance of simple inorganic chemicals spewing out of volcanoes into the hot seas and atmosphere (for us it would be deadly and briefly(!) very smelly too). The good news, though, is that some of those simple chemicals are fine electron donors and some others are good electron acceptors. By harvesting a donor-acceptor pair and letting them react, our primitive bacteria can free energy and let it flow through them. Many Aquifex today, living in hot springs (around 90ºC), exploit hydrogen gas and dissolved nitrate as donor and acceptor chemicals respectively. Notice that neither of these is organic in origin: organisms that derive energy from inorganic chemicals are lithotrophic (literally rock eaters). Aquifex are also anaerobic, as we shall see shortly. Both these traits would have been needed by early life.
*There are other strong contenders for this position, notably the hyperthermophilic Crenarchaeota, which are Archea rather than bacteria. In particular these include species having hydrogen sulfide (H2S) as an electron donor and iron sulfide (FeS) as an acceptor, both chemicals abundant in hydrothermal vents.
Indeed these may have coexisted with Aquifex species, not least because the reaction
FeS + H2S -> FeS2 + H2, produces the hydrogen gas typically used by primitive bacteria to reduce nitrate. These days, Aquifex and various Creanarchaeota species live together, for example in the near-boiling volcanic springs of Iceland.
Indeed these may have coexisted with Aquifex species, not least because the reaction
FeS + H2S -> FeS2 + H2, produces the hydrogen gas typically used by primitive bacteria to reduce nitrate. These days, Aquifex and various Creanarchaeota species live together, for example in the near-boiling volcanic springs of Iceland.
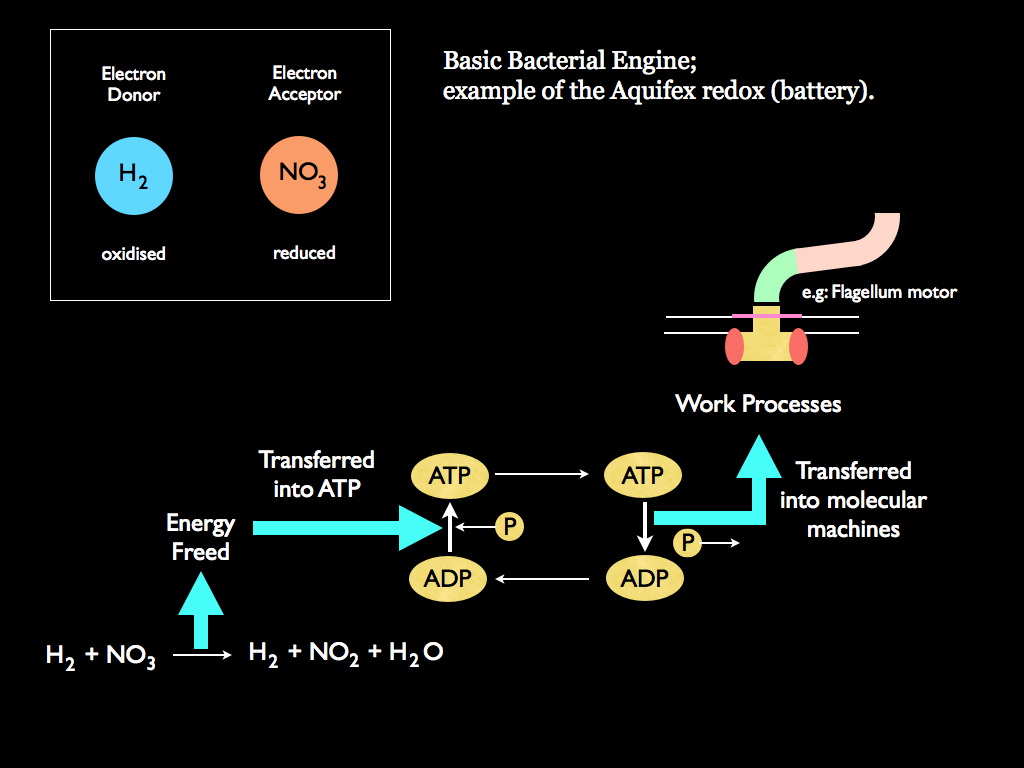
So primordial cells need an electron donor / electron acceptor pair to free energy which can then flow through their bodies, which really means across membranes and through biochemical reaction chains (pathways). The bare bones of this energetic process is illustrated below (noting that other donor/acceptor pairs are available). The energy freed by the reduction / oxidation (redox) reaction is transferred via either fermentation or respiration into the synthesis of energy carrying ATP (Adenosine Triphosphate) by means of adding a third -phosphate (PO3) to Adenosine Diphosphate (ADP), which obviously has two of them already (this addition is called phosphorylation). These ATP molecules act in a way analogous to the current in an electric circuit (and even more analogous is the oxygen carrying red blood cells, but let's not get confused by that). A healthy living cell is filled with ATP molecules that get everywhere within it, which is just as well because the ATPs are used by every process of the cell that needs to pump entropy into passing energy. The third phosphate attached in ATP acts as a chemical constraint on a little 'package' of energy: this bound-up energy is ready to accept some entropy from what ever chemical reaction can couple to the ATP, then release the third phosphate (by hydrolysis) and thereby free the energy, which immediately takes a lower grade - higher entropy - form. (Note conventionally, this is described as transferring energy from ATP to a coupled process, but you know I am keen to emphasise the underlying entropy accountancy here, so think of ATP as carrying some energy, which in turn carries some entropy).
When the energy is released by removing the third phosphate, leaving ADP behind, it is released in a constrained system hence it is thereby able to do some work (the transfer of entropy). An obvious example of this is the driving of a flagellum motor, which is a natural nano-machine made from several large protein molecules assembled in a very particular way, so that when they get an injection of energy from ATP, one set moves a step around the other and an attached axle (yet another protein molecule) turns, which twitches the flail that works like a little propeller enabling the organism to swim. So the flagellum motor is literally a little engine that does mechanical work by pumping entropy into energy that passes through by changing from chemical to mechanical form. Well, the flagellum motor is just an obvious example; ATP supplies energy to a huge range of working parts, including signaling systems (which require energy to do the work of communication, just as your internet modem / WiFi does), molecular assembly (e.g. to put together those flagellum motors), and of course, ultimately for reproduction. The ADP molecules return to the part of the organism where ATP is synthesised (essentially they just diffuse over to pick up some more energy carrying phosphate).
The place this happens is in a membrane, specifically, in the catalysed chemical reactions taking place among embedded transmembrane proteins. As with all prokaryotes (bacteria and archea), there really is only one membrane, the tegument or skin of the organism, so our Aquifex capture their energy in their skin (in their case, not by respiration, but by the anaerobic alternative of fermentation). Indeed, the analogy I made early with an electrical battery gets even closer to reality since the process of creating ATP involves charging the membrane positive (excess of hydrogen ions) on the outside, resulting in a negative charge on the inside: literally making the skin a little battery. Connected to this, a nano machine (embedded in the membrane) called ATP synthase actually does the work of adding phosphate to ADP molecules. The ATP / ADP act as the current, delivering energy to many different nano-machines that do the work of autopoiesis and move the organism around. The thermodynamic cycle is completed by the returning ADP: low entropy energy is picked up from the redox reaction, put into ATP by ATP synthase, which runs off the electrical potential created by the redox reaction, the ATP carries this low entropy energy to a range of coupled systems where its transfer is constrained to do work (gaining entropy), via hydrolysis of ATP to give back ADP and free phosphate, these diffusing back to the ATP synthase machine to repeat the cycle. You can compare this to diagram B above and see that the redox reaction is analagous to the fire, the ATP synthase is roughly analagous to the hot tank, the flagellum motor equivalent to the piston and wheel, and ATP / ADP are equivalent to the hot and cold gasses.
Every cell performs essentially the same thermodynamic cycle with equivalent components and systems. Once free oxygen became abundant (as a by-product of oxygenic photosynthesis in the cyanobacteria, who used this instead of inorganic redox reactions), aerobic respiration became a more efficient option than anaerobic fermentation. With life abundant and all sorts of biomolecules around, feeding off chemical reactions involving these became an efficient alternative to lithotrophy and bacteria evolved that could aerobically capture energy from oxidising the likes of sugars. Some of these were probably incorporated (literally made part of the body) of some archea species, where they would gradually loose all but the most essential of functions and become obligate endosymbionts (internal symbiotic quasi-organisms entirely dependent on their hosts). These were the mitochondria which now act as the little engines in every eukaryotic cell.
Work and Life
Life is only possible if it can do work (in the technical sense used in physics). The textbook definition of work in this technical sense means the result of a force acting over a distance in a particular direction and you might ask what that has to do with living. The answer is that in thermodynamic equilibrium, matter consists of a lot of particles moving in random directions, hence with random momentum and this results in forces (for example gas pressure) that have no coherent pattern to them, so that on average (and we are averaging over huge numbers of particles) forces balance, so there is no net movement. This lack of net movement is a result of incoherence, of randomness and therefore of lack of pattern and therefore lack of embodied information. There is kinetic energy in all the incoherent movement, but in this random configuration, it cannot yield any work. For that we need some coherence to the momentum of the particles and this is achieved by limiting their scope, that is by constraining them (for example with a chamber and piston), which of course is equivalent to reducing the entropy of the system. Work is possible when there is a net direction to forces and this necessarily implies some organised pattern to the movements. Organised pattern, in turn, implies information. Once energy is organised into some information instantiating pattern, that pattern-information can be transferred to another component of the system. A thermodynamic work cycle (referring again to Stuart Kaufmann’s observation) is a process of extracting work from organised energy by transferring the information of organisation into another form, or part of the system. So, for example, constructing a protein molecule is an act of organising matter and to do it, life must sequester that organisation from energy. It is in this sense that catabolism (making body parts like protein molecules) uses energy. The energy is not used up (the First Law of Thermodynamics ensures that energy is never created nor destroyed); rather energy is disorganised, taking the information out of it to use in the catabolic process. The energy of photosynthetically active radiation (e.g. red and blue light) is more organised that that of heat radiation, so in transforming sunlight into warmth, plant leaves are supplying the necessary ‘organisation’ for constructing plant body parts. This ‘organisation’ is the information embodied in organic molecules: first in sugar (as a sort of currency), which is then transferred into a wide variety of molecules and structures built from them. Whenever energy is converted from a ‘high grade’ such as light to a ‘low grade’ such as heat, work can be extracted and used to construct something or perform a coherent action. Since living consists of a set of coherent and highly organised chemical reactions, many of which are also catabolic, there is plenty of need for work. Life would not exist without it.